Nine key considerations for CROs seeking to improve operational efficiency, improve visibility, and meet deadlines — and how Runn supports those strategic objectives.

Clinical Research Organizations (CROs) operate in a highly specialized, high-stakes environment where resource precision, real-time visibility, and accurate planning are critical.
Runn’s resource management software is best placed to address these challenges, helping you manage resources efficiently, get the most out of your people’s time, and deliver successful trials on tight deadlines. You’ll also find reporting tools that deliver actionable real-time insights across your entire organization so you can confidently make quicker, better decisions.
To run efficiently, profitably, and sustainably, CROs must strike the right balance between the cost and value of their human resources. Highly skilled specialists are a CRO’s greatest asset — but when underutilized, they become a draw on costs. On the other hand, pushing staff too hard risks burnout, decreased performance, and costly turnover.
With limited hours, niche expertise, and increasing trial complexity, CROs must make the most of resources. That means making sure every team member is contributing at the highest value possible, without compromising well-being or quality of work. It's a difficult balance to maintain, especially when managing multiple overlapping trials across different therapeutic areas, but it’s one that can be achieved with the strategic use of purpose-built resource management tools.
Further reading: 6 Resource Management Best Practices for CRO Success ➡️
Clinical trials frequently experience shifting timelines due to funding changes or regulatory requirements. Runn’s flexible scheduling capabilities allow you to easily adjust resource plans in real time, ensuring your team can pivot quickly if milestones are changed, trials are delayed, or additional resources are suddenly required.
.png)
Yes, Runn seamlessly supports blended workforce planning. Whether you're working with full-time employees, contractors, or part-time consultants, Runn allows you to manage all these employment types within a unified resource management interface, ensuring optimal allocation and utilization.
Definitely! Runn's Project Planner clearly visualizes overlapping projects, allowing you to manage multiple trials simultaneously without resource conflicts. You can easily drag and drop resources to adjust allocations, preventing bottlenecks and ensuring all your trials progress smoothly.
Yes. Runn supports centralized resource planning by providing a central source of truth, helping dissolve departmental silos. You can view real-time staff availability from across departments in one place, ensuring comprehensive visibility and enabling swift decision-making to meet tight clinical trial deadlines.
Dig deeper: Why Resource Visibility is Essential for Clinical Research Organizations ➡️
Runn includes advanced filtering and custom fields that enable precise matching of specialized experts to specific trial requirements. In Runn, you can add skills to your people, with no limit to how many skills there are across your company and no limit to how many skills a single person has.
Using skills filtering, you can quickly find and allocate principal investigators, regulatory specialists, or therapeutic-area experts, ensuring the right talent is matched to the right trial at the right time.
Further reading: Learn how to search for people and projects with Runn. ➡️

CROs operate within complex, demanding environments where precision, efficiency, and agility are critical. Runn simplifies and optimizes a range of resource management tasks, enabling CROs to effectively handle multiple overlapping trials and complex demands. These include:
See it in action: Learn how to manage and use skills in Runn ➡️
Runn empowers CROs to operate with greater clarity, control, and confidence in an industry where timing, precision, and efficiency are everything. Designed to meet the fast-moving, high-stakes demands of clinical research, Runn helps organizations:
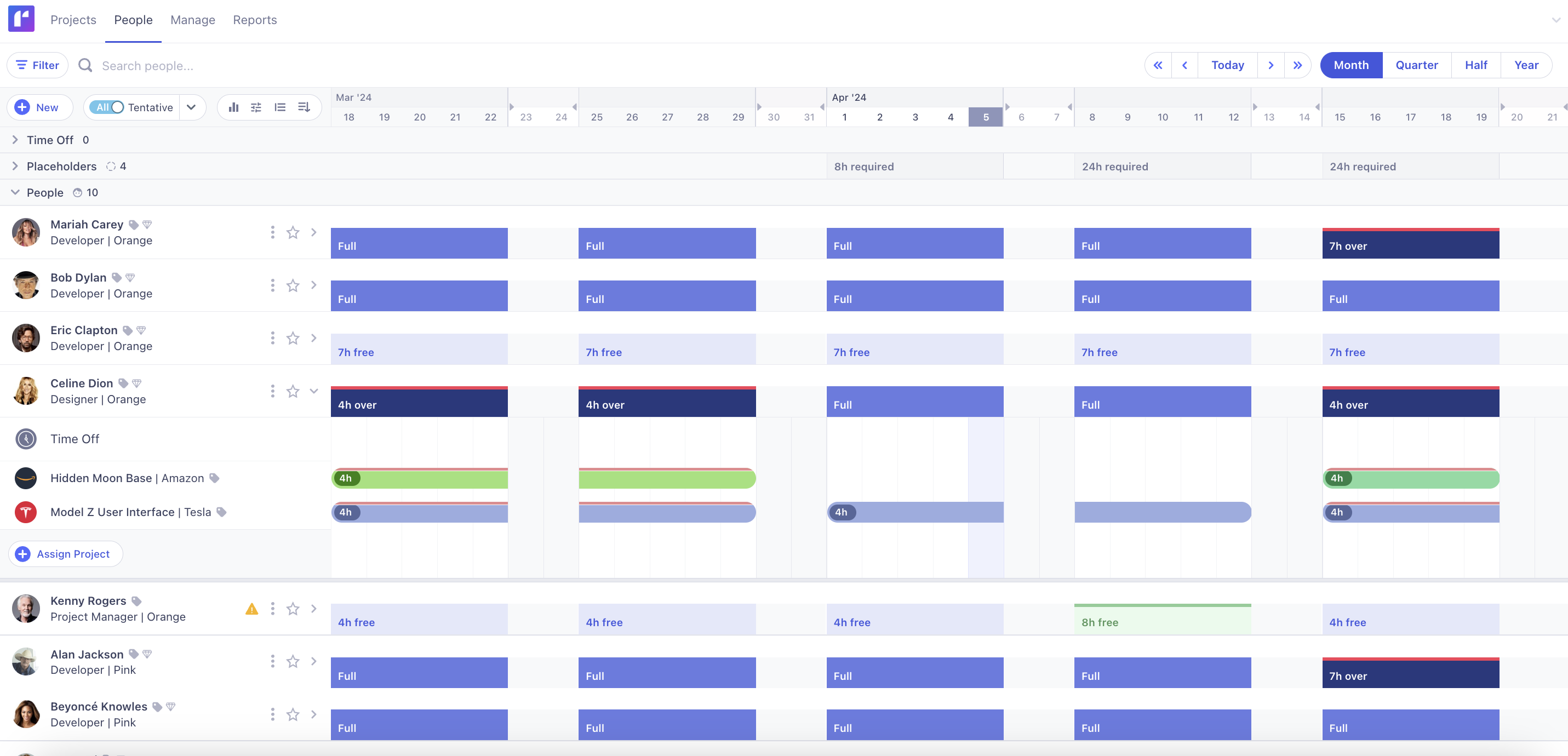
Runn is purpose-built for the fast-paced, high-stakes environment CROs operate in. Unlike generic tools, Runn is tailored to the unique demands of managing multiple concurrent trials, tight timelines, and highly specialized teams.
Runn gives CROs the tools to stay agile, efficient, and trial-ready, no matter how complex the landscape becomes.